What Is Purified Water (PW), and Water for Injection (WFI)?
Water is the most critical ingredient in any pharmaceutical plant and, when used for production at any volume, is highly regulated. All pharmaceutical waters in the United States are subject to Food and Drug Administration (FDA) and current Good Manufacturing Practice (cGMP) guidelines for the removal of minerals, organics, and microbes, even when water does not remain in the finished product.
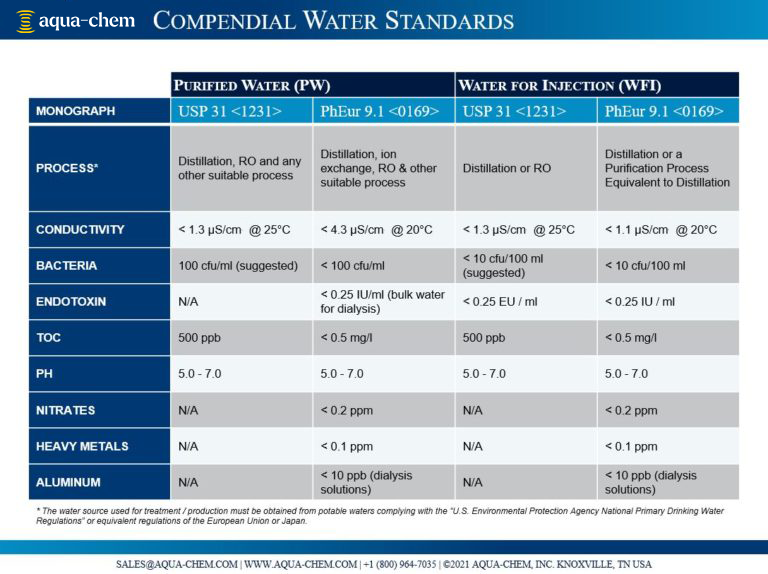
Regulatory bodies, such as the United States Pharmacopeia (USP), European Pharmacopeia (PhEur or EP), and Japanese Pharmacopeia (JP), write and regularly update ‘Monographs’ that outline the standards for identity, quality, purity, and handling of water and other ingredients used for pharmaceutical production. Waters that are compliant with monographs are described as “Compendial” and currently fall into two broad categories; “Purified Water” (PW) when produced for oral or external use and “Water for Injection” (WFI) when produced for parenteral (injectable) use.
Compendial water treatment must always begin with a potable water source before one or more treatment steps remove minerals, organics, and microbes to defined levels. The specifications for WFI are more stringent than PW, and some manufacturing sites add additional requirements that exceed that of their local regulatory body. The equipment used for water production must be carefully validated to confirm proper installation and operation and is rigorously monitored for performance and adherence to quality specifications.